The control panel is ergonomically designed and positioned at the optimum viewing angle for the user. Users can operate easily with any posture. Since the panel is pressure-sensitive, the device can be operated with gloved fingers or a stylus pen. The stylus pen can be stored right next to the control panel.
Easy-to-Use Interface Grasp the Current Status and Operating Procedures at a Glance
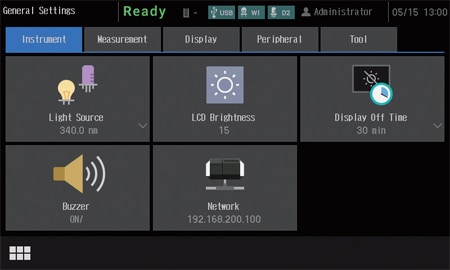
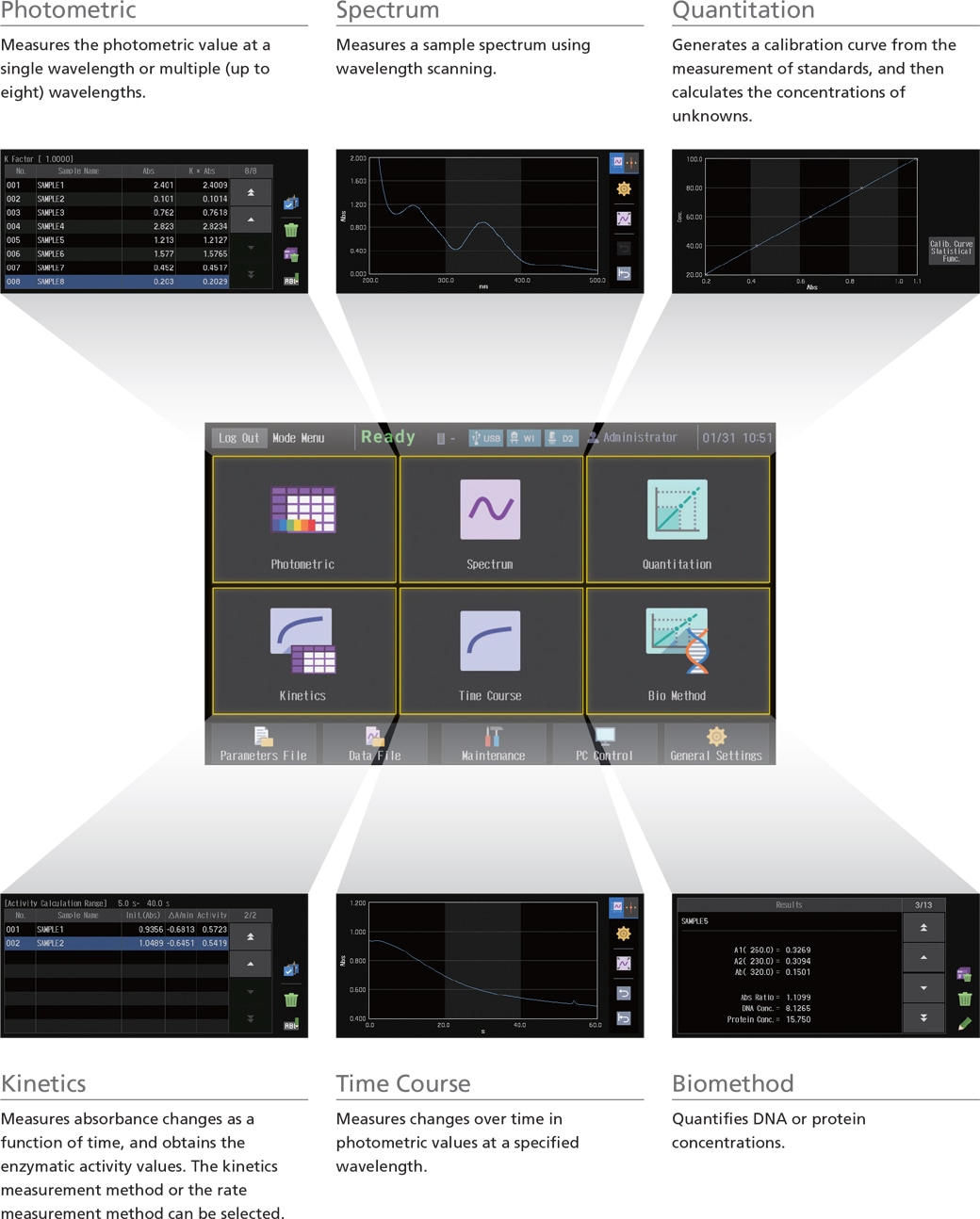
The UV-1900i on-screen user interface includes large, easy-to-see icons deployed on a black background, so the instrument settings are evident at a glance. In addition, the large, easy-to-see icons improve intuitive understanding, which enables users to quickly become familiar with the operations. Furthermore, the user interface is designed to minimize transitions between windows, so users do not get confused during the operations.
Navigation Tabs Improve Usability
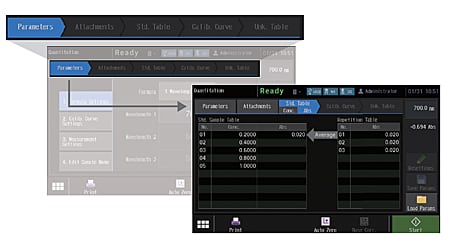
In quantitation mode on the UV-1900i, the stages of the entire measurement process and the current status are always shown on the display. As a result, users know immediately what to do in the next step.
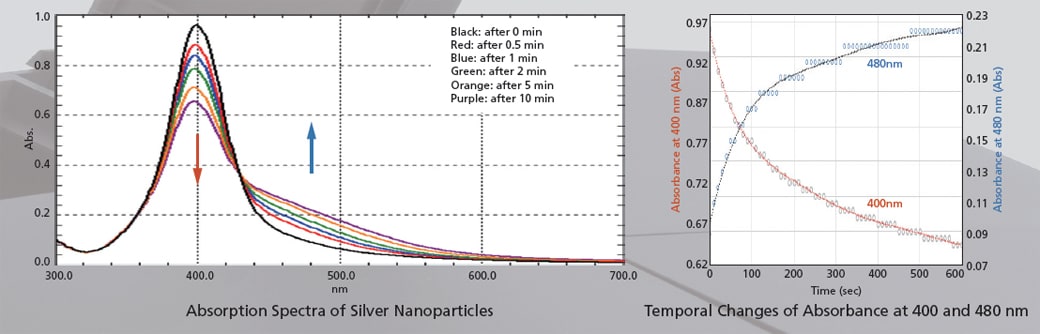
Therefore, more detailed behavior can be investigated by observing spectra with the UV-1900i.
The figures below show the analysis of the particle agglomeration process when salts are added to silver nanoparticles. Measurements of the 300 to 700 nm region were performed in ultra-fast scan mode. In addition to the decrease of absorbance at 400 nm and the increase of absorbance at 480 nm, the temporal changes of spectra can also be observed.
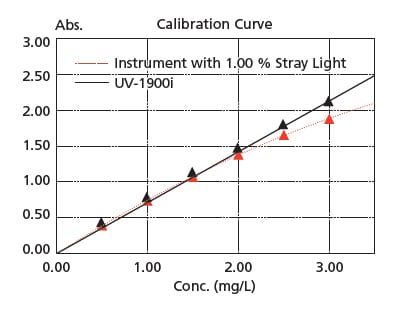
Stray light is at 0.5 % max. (198 nm), making accurate measurements are possible up to the vicinity of 2 Abs even in the ultraviolet region. In addition, high-concentration samples can be quantified accurately.
The figure on the right is a calibration curve for acetic acid, created with absorbance at 200 nm. The correlation coefficient is 0.9997 and correct measured values are obtained even in the vicinity of 2 Abs. Linearity will be lost in the high absorbance region due to the stray light.

The figure on the right is a calibration curve for caffeine, created with absorbance at 273 nm. The calibration curve has an Abs = 0.0528 Conc. The lower limit of quantitation determined from the standard deviation is 0.0051 mg/L.
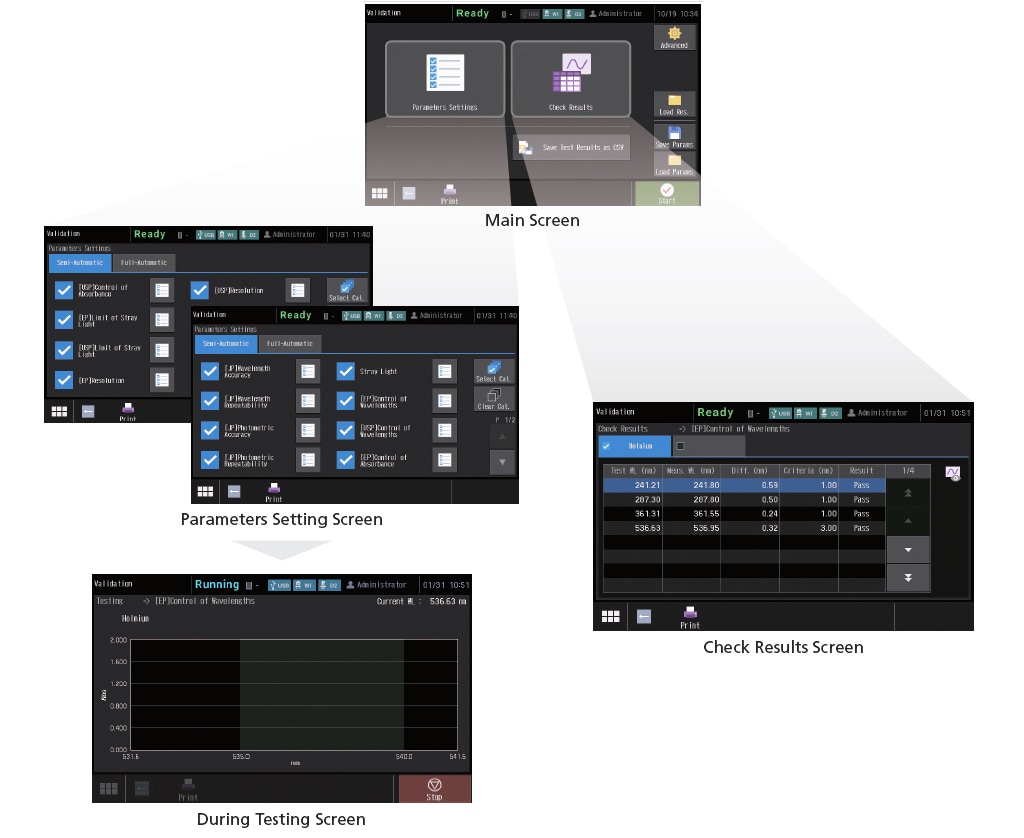
This instrument can not only run checks for nine JIS items, but also those stipulated in the Japanese Pharmacopoeia (JP), United States Pharmacopeia (USP), and the European Pharmacopoeia (EP). Naturally, the hardware is also compliant with the specifications required by each Pharmacopeia. In addition, the check conditions can be saved. As a result, once the conditions are saved, checks can be performed easily just by calling them up as needed. Check results can also be saved.
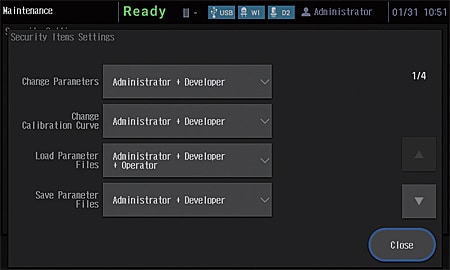
An external control security function has been added to provide more support for compliance with regulations. Three user authority levels, "Administrator", "Developer", and "Operator", can be set for instrument users.
In addition to achieving a resolution of 1 nm, the highest in its class, by using a monochromator with a Czerny-Turner mounting, the UV-1900i also features a compact, bright optical system. The instrument is more than capable of meeting the wavelength resolution required in the European Pharmacopoeia.
Validation can be implemented with PC software by using the optionally available UV validation software.
In addition to simplifying daily inspections, this makes instrument performance checks and records management easier, enabling more secure regulatory compliance.
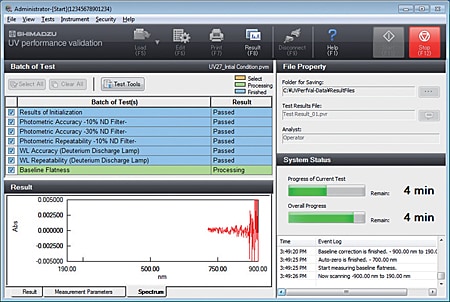
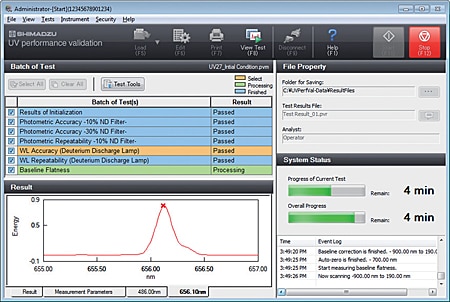
Ensuring the integrity of data (database management), including the user management, user authority management, and data audit trails required for compliance with FDA 21 CFR Part 11, PIC/S GMP guidelines, and other ER/ES regulations, is possible.
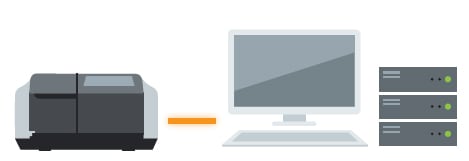
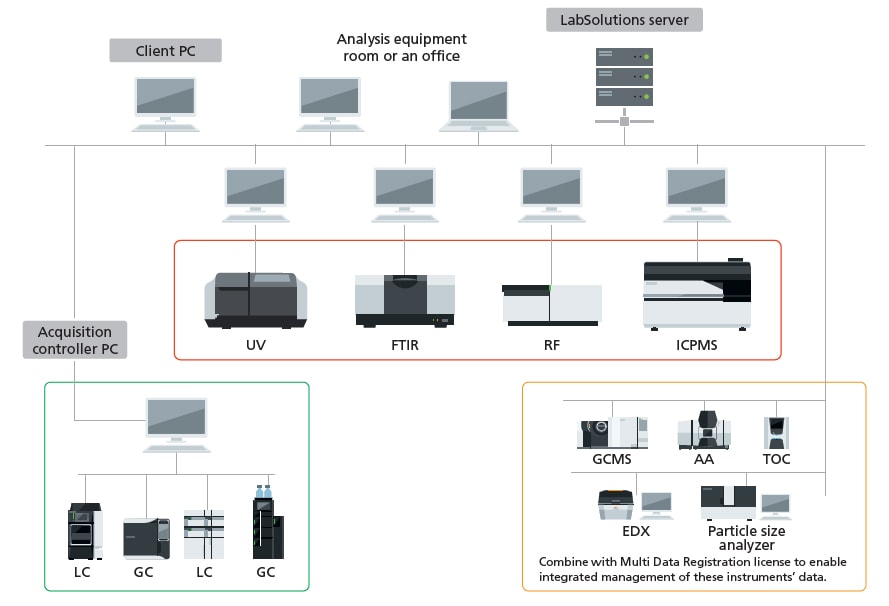
The system allows for data management and user management with a database. Compliant with ER/ES regulations, the system is optimally configured for customers using a PC.
The system is optimally configured for customers who want to manage data on a server together with LC and GC data for ER/ES compliance.