The Japanese Pharmacopoeia 17th Edition (JP17) and the United States Pharmacopeia (USP) General Chapters <467> Residual Solvents provide test methods for residual solvents in pharmaceuticals mainly using headspace gas chromatography (GC). Residual solvents in pharmaceuticals are classified from Class 1 to 3 based on their potential human health risk and are strictly controlled. The list of residual solvents is continuously reviewed and methylisobutylketone (MIBK) has been newly added to JP17 Supplement II in 2019. The compound is classified as Class 2 mixture A standard solution (Class 2A) in the USP. In this article, MIBK was analyzed and its chromatogram was overlaid with that of Class 2A as a reference.
Shimadzu HS-20 headspace gas sampler was connected to NexisTM GC-2030 gas chromatograph and used to measure Class 2A and MIBK standard solutions as described in JP17 Supplement II. As the standard solutions, water-soluble and water-insoluble samples were prepared and measured by Procedure A and B, which employ different types of columns, column temperatures, and split ratios. Table 1 below list the analysis conditions for water- soluble samples respectively.
Fig. 1 and 2 show the analysis results by Procedure A and B respectively. (Black: Class 2A, Pink: MIBK)
* The resolutions in the Fig. 1and 2 are reference values and not
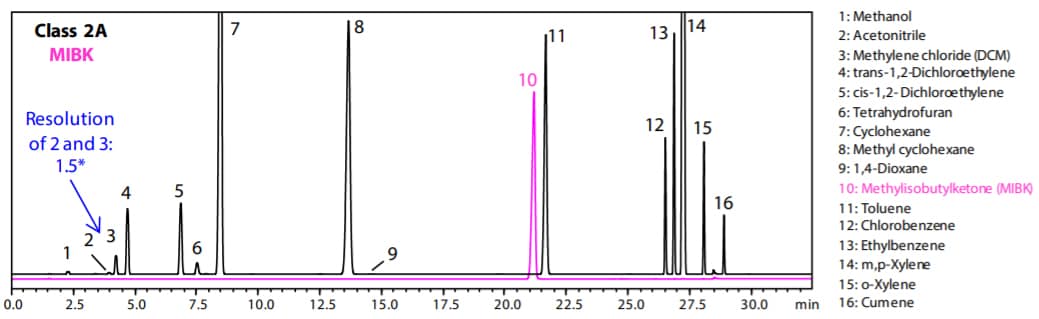
Fig. 1 Chromatogram of Class 2 A and MIBK Standard Solution by Procedure A (Water-Soluble Sample)
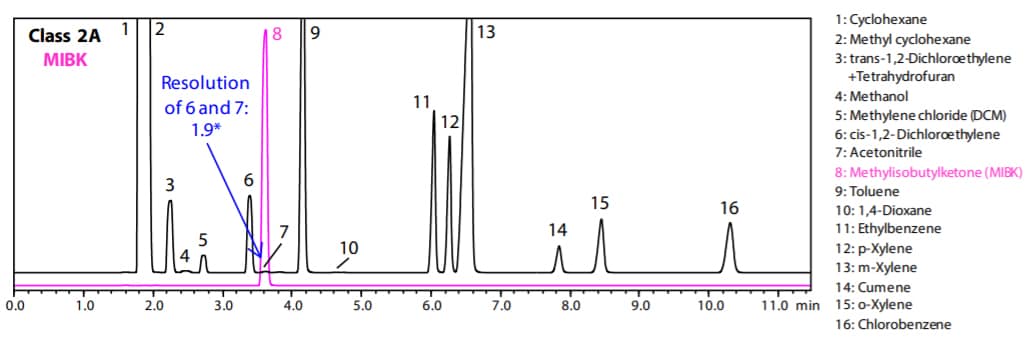
Fig. 2 Chromatogram of Class 2A and MIBK Standard Solution by Procedure B (Water-Soluble Sample)
GC analysis conditions (Procedure A and B)

In combination with a robust GC detector, the system can be used for quality control requiring high quantitative accuracy. Traceability is guaranteed by the all-sample leak check function and the digitized log function.

