■ Analysis of Trace Impurities in Atropine
The FDA draft guidance, "Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches," addresses the verification of the genotoxicity and carcinogenicity of impurities in pharmaceutical ingredients, which is not covered in the ICH guidelines. Impurities with possible or unknown genotoxicity contained in new drugs, investigational new drugs, and generic drugs must be restricted to a daily intake of 1.5 µg max, assuming that the person takes the drug over 12 months.
Structural analysis of the impurities is required when verifying the genotoxicity and carcinogenicity of impurities and when improving the manufacturing processes to keep the daily intake to 1.5 µg max. This threshold value demands structural analysis of trace impurities in a 200 mg daily drug dose at levels approximately 150 times lower than prescribed in the ICH guidelines.
NMR is a method for the structural analysis of organic impurities. However, due to the need to analyze mixtures and the demand for sensitivity, LCMS-IT-TOF mass spectrometry, which offers exact mass measurements and MSn, is an effective method for the structural analysis of ultratrace impurities.
The analysis of trace impurities in atropine is introduced below.
The analysis results of commercially available atropine spiked with 0.001 % p-nitrophenol trace impurity are shown below.
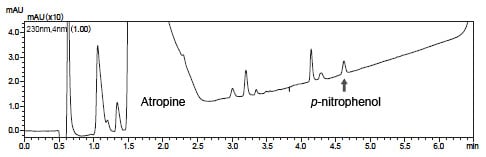
Fig. 1 shows a magnified view of the LC chromatogram. The arrow indicates the peak of the spiked p-nitrophenol.
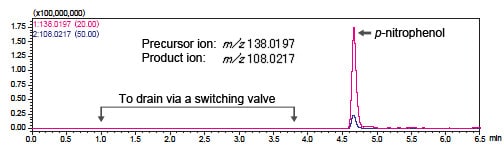
Fig. 2 shows the LCMS-IT-TOF analysis results for the p-nitrophenol peak.
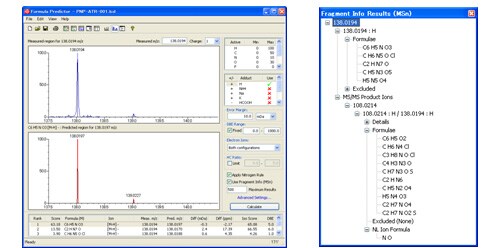
Fig. 3 and Fig. 4 show the results obtained by using the formula prediction software on the obtained MS and MS/MS values

• A high-resolution mass spectrometer for structural analysis, it uses unique rapid positive/negative ion switching to dramatically increase the information acquired from a single sample injection and significantly accelerates decision-making for formula and structure predictions.
• The Dual-Stage Reflectron (DSR), Ballistic Ion Extraction (BIE), and precise temperature control of the flight tube allow the acquisition of high-accuracy MSn data over a long period of time. This mass stability permits accurate impurity formula and structure predictions.
• A neutral loss survey performs MS3 measurements using the desorption of specific neutral molecules obtained in the product ion spectrum as the trigger and MS2 measurements using a specific isotope pattern as the trigger. These automated MSn functions, based on the structural similarity of the compounds, only evaluate the ions structurally related to API.
The workflow for the process from impurities detection to formula/structure prediction is significantly accelerated by two pieces of software: MetID Solution, which uses the similarity of the MSn spectra to automatically extract peaks with structural relativeness to API; and Formula Predictor, which not only uses mass accuracy for structure estimation but combines isotope pattern scoring, chemistry rules, and MSn filtering.

